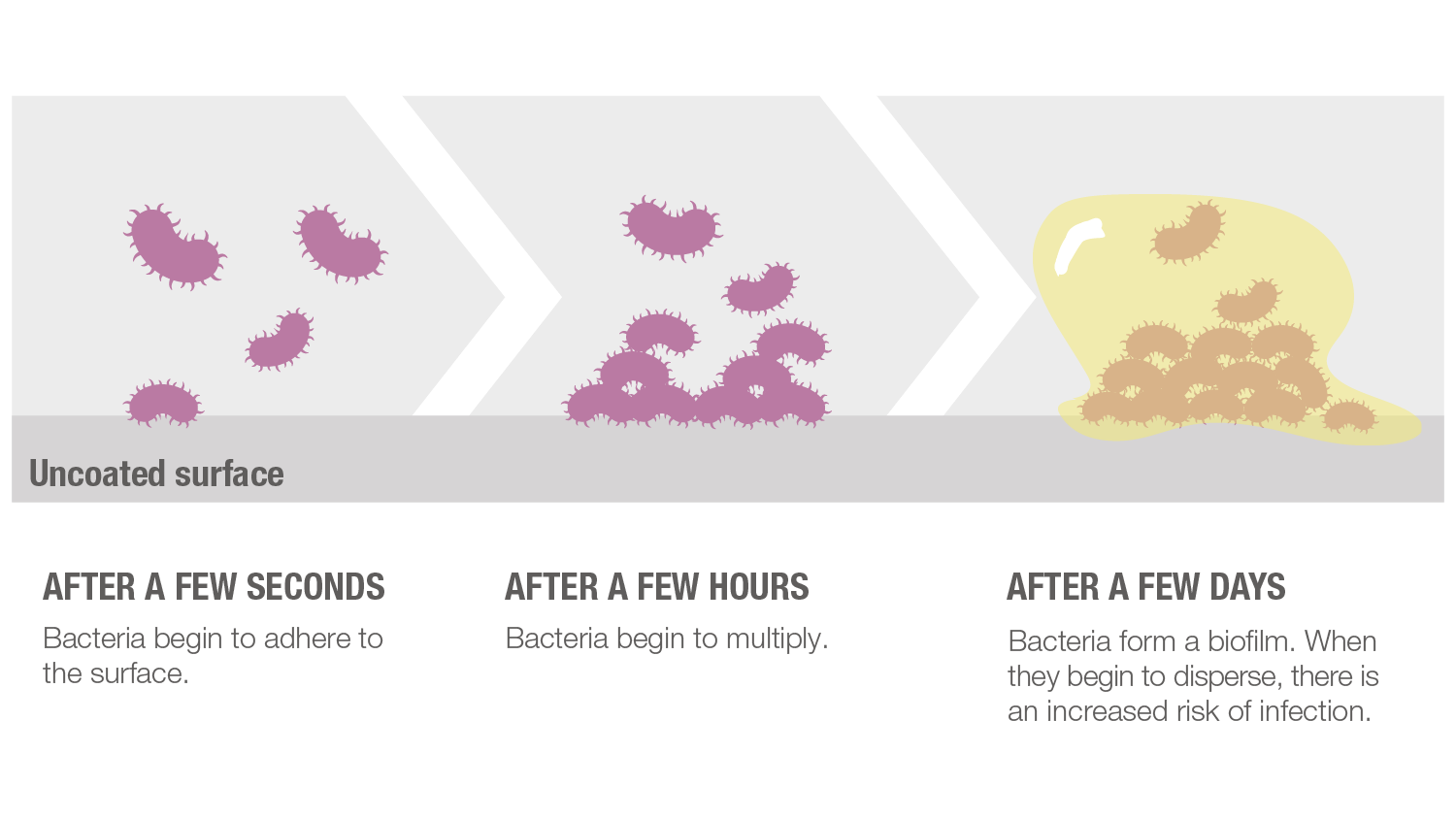
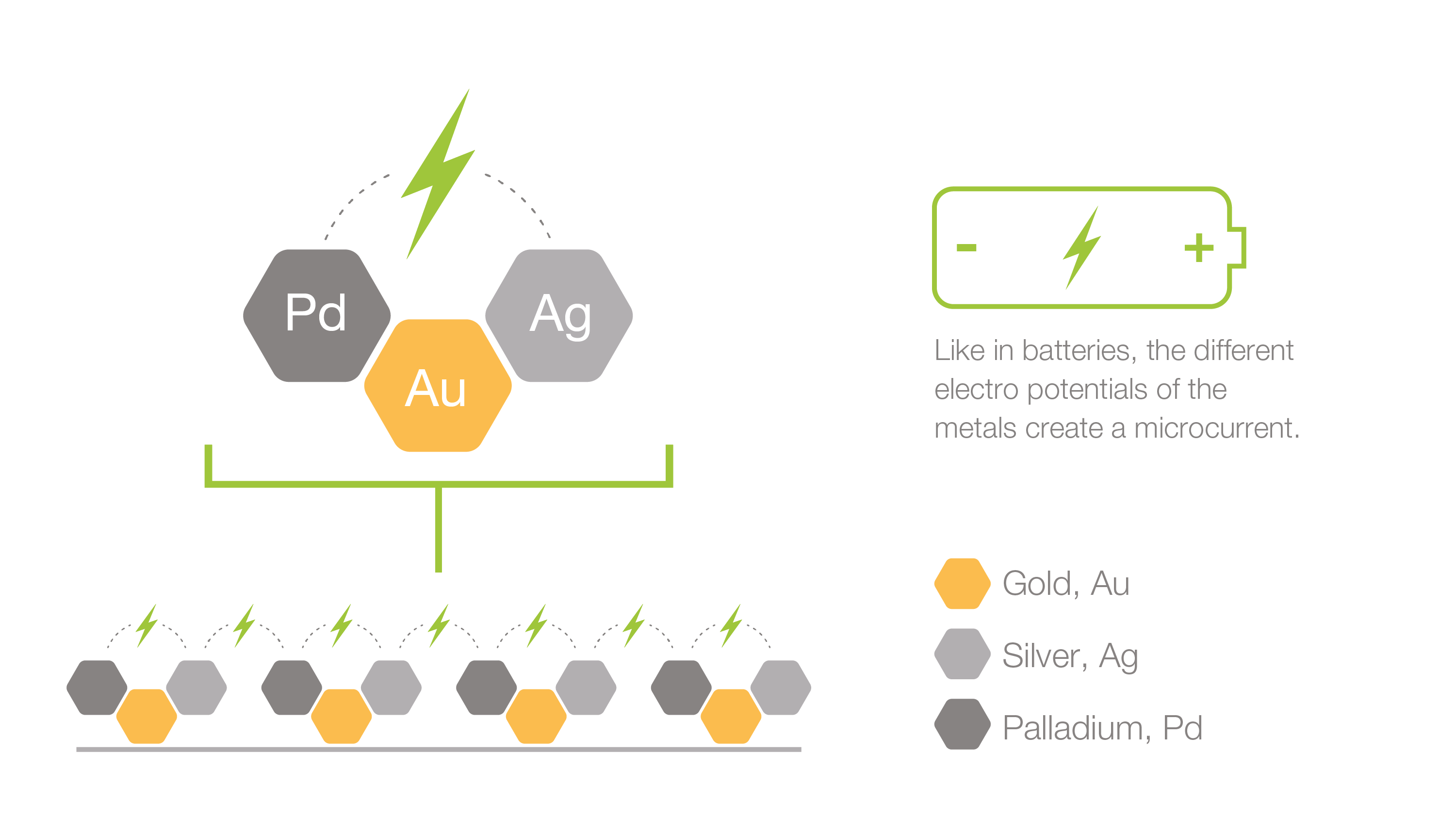
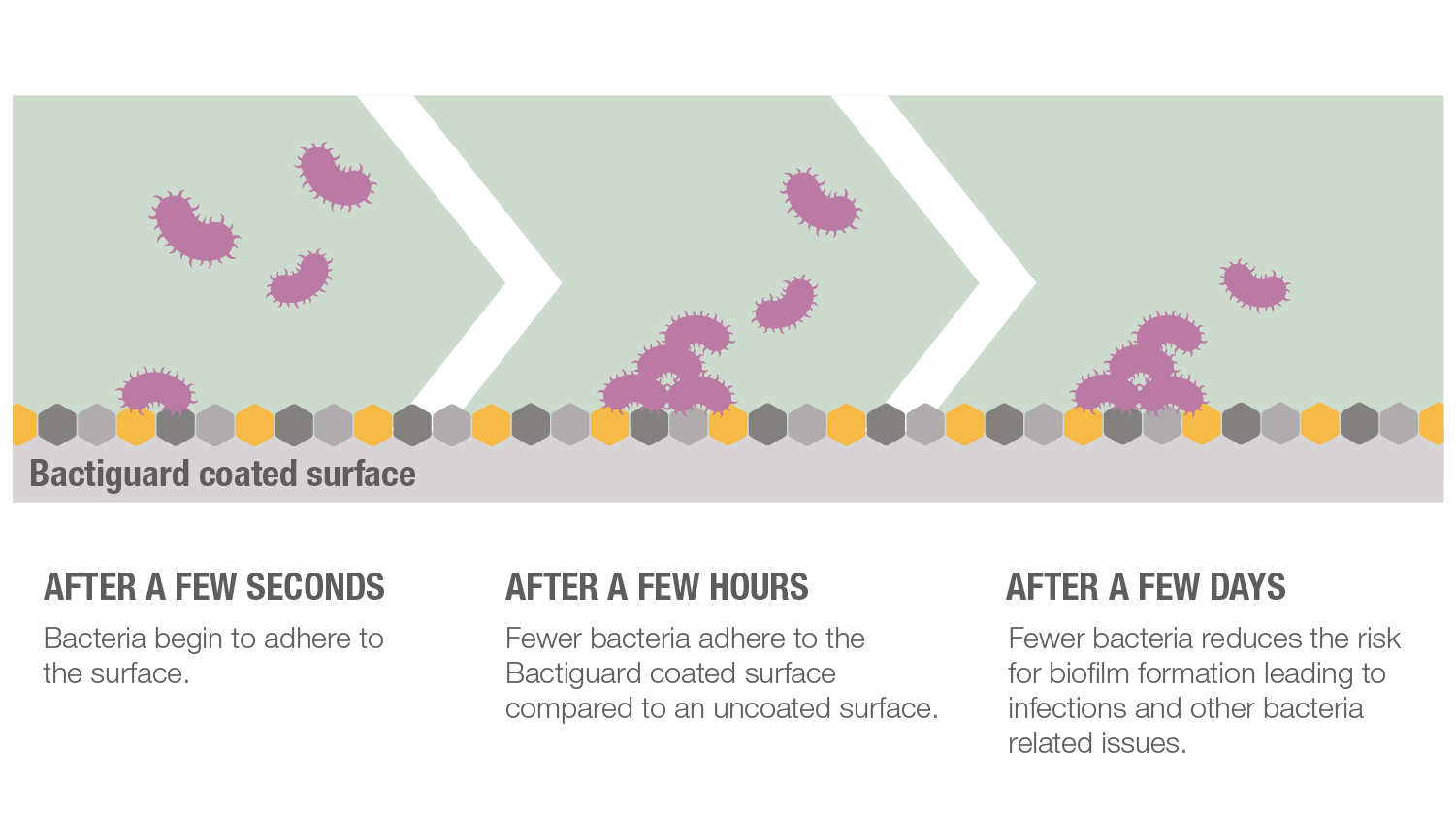
The amount of noble metals at the surface is very low and below all safety limits for each metal, and there is no release of any toxic or pharmacological quantities. This makes the technology both tissue-friendly and safe as opposed to traditional coating technologies which often depend on the release of substances that kill bacteria, e.g. high concentrations of silver ions, chlorhexidine or antibiotics.
Our technology is approved for long-term use on orthopedic trauma implants and can thus be used on implants that will remain with the patient for life. To date, more than 230 million Bactiguard coated products have been sold for patient use, with no reported adverse events related to the coating.
There are no specific procedures for disposal of Bactiguard coated products. The noble metals are trapped in special filters at the incineration plant.