Bactiguard’s focus is infection prevention. Revenues are generated by our own product portfolio and license business. Bactiguard’s offering is an important contribution to the sustainability efforts of reducing the risks of healthcare associated infections.
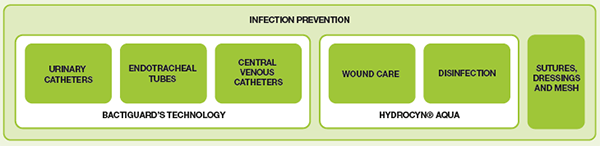
Until February 2020 our business comprised Bactiguard’s unique technology, which we license to leading medical device companies globally, and offer in our own range of products. The acquisition of the Malaysian company Vigilenz in 2020 expanded our range with a portfolio of products for wound care and disinfection, Hydrocyn aqua, as well as sutures and other wound care products.
License business
We license Bactiguard’s technology to leading medtech companies that apply our technology to their own products and sell them under their own brand. This license business gives us access to a large global market, while making our technology available to as many as possible.
In our license business we receive initial fees related to the right to use Bactiguard’s technology for products within a specific application and geographical area. The license revenues also comprise royalties; which is a variable remuneration when the products reach the market and generate sales revenues. The licensees gain access to Bactiguard’s expertise in technology, production and regulatory approval processes. We also supply them with the coating itself; a concentrate from noble metals.
BIP portfolio
We call our own product portfolio ‘BIP’ (Bactiguard Infection Protection). The portfolio contains urinary catheters, endotracheal tubes, central venous catheters, Hydrocyn for disinfection and wound care, as well as sutures and other wound care products. Our products prevent infections, and are effective and biocompatible.
We sell our product portfolio either directly or through distributors across of the world. When we sign a new distributor agreement, it normally takes between one and one and a half years before the collaboration starts to produce tangible results. The amount of time required depends, for example, on national regulatory approval processes.
The acquisition of Vigilenz gave us access to a well-established sales organisation in Malaysia. In 2020 we took the strategic step to build our own sales organisation in the Nordic region so that we can approach our domestic market more effectively.
Our business model