The Swedish MedTech Regulatory Summit in Stockholm stands out as a significant event in the MedTech sector. Since its establishment in 2018, the Regulatory Summit has grown to become the annual conference with a focus on medical device regulations. Interest in the subject has only amplified over the years, reflecting the industry’s commitment to advancing regulatory processes, including Market Access, CE marking, clinical research for new devices vs practicability, post-market clinical studies, and more.
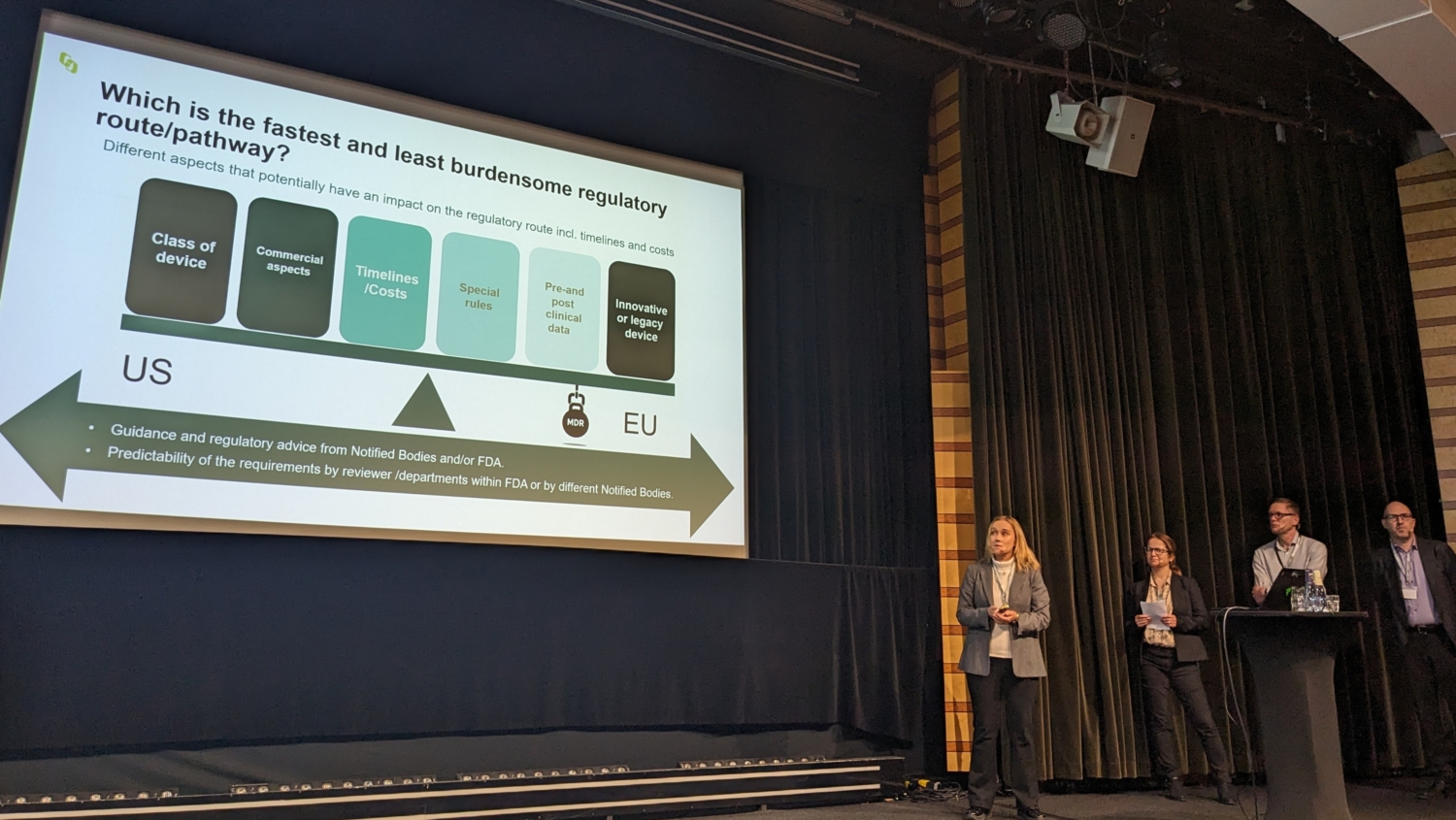
Bactiguard was represented by Chief Quality and Regulatory Officer Fatima Stensvad Flodin. Fatima focused on “EU som första marknad” (EU as the first market), discussing the intricate dynamics of choosing the fastest and least burdensome regulatory route for medical devices. Amidst the evolving regulatory landscapes, Fatima’s discourse was particularly relevant for stakeholders grappling with the complexities of regulatory pathways in the EU.
Fatima’s fellow panelists included Karl-Yngve Keck from Getinge, Sebnem Yavas Hoffsten from Medtronic, and Peter Löwendahl, Senior Consultant at Hoff&Lowendahl AB and chair of the Swedish Medtech’s focus group Regulatory Affairs. Their collective expertise provided invaluable perspectives on how to successfully navigate the regulatory landscape in Europe and what it needs to evolve to cement its status as the second-biggest market in the world.
At the heart of the summit, there was a unified understanding of the crucial role the EU market plays for MedTech companies and the imperative for these companies to skillfully maneuver through its regulatory landscape. A key point of discussion was the need for regulatory processes in Europe to be streamlined into a simpler, less complex, and more centralized approach. This adjustment is particularly important to ensure that innovative companies can establish and maintain their platforms within Europe.
The summit wrapped up on a progressive note, underscoring the significance of cooperation, the sharing of knowledge, and the drive for innovation as pivotal forces in sculpting the future of the MedTech industry. As regulatory frameworks are in a state of flux, entities like Bactiguard are not merely navigating these transitions but are also laying down exemplary benchmarks for patient safety and the quality of care. This proactive engagement with the evolving regulatory environment demonstrates a commitment to fostering an ecosystem that supports innovation while upholding the highest standards of patient care.